Sea Stars Feed on Marine Mollusks Survivorship Graph
- Original Article
- Published:
Cohort life tables for a population of the soft-shell clam, Mya arenaria L., in the White Sea
Helgoland Marine Research volume 69,pages 147–158 (2015)Cite this article
Abstract
The Mya arenaria generation in the White Sea was observed for almost the whole life cycle (around 25 years). Using the data on this generation dynamics, the cohort life table was built. The main purpose of the research is analysis of age-specific mortality in this soft-shell clam population. The mortality rate was found to change more than tenfold throughout the study period. No regular changes in its value were observed. Periods of low mortality alternated with periods of much higher mortality. Several reasons for the increase in M. arenaria mortality rate during its life cycle are proposed: (a) living in the surface sediment layer at early stages of life cycle (unstable environment, high mortality of non-viable clams, predator impact); (b) intense intraspecific relationships in dense aggregations of young clams; (c) increased intraspecific competition during periods of the rapid individual growth; (d) ageing (clam mortality increases with older age—achievement of an average and a maximum lifespan).
Introduction
Populations and individual age groups are dynamic due to two main processes—the death of individuals from different age classes and the birth of new individuals. The intensity of these processes depends on the age of organisms. A convenient way to report the survival of different age individuals and the contribution of individual age classes into the population reproduction (the age-specific fecundity) is constructing life tables.
By now, the life tables have been produced for a number of benthic marine species: the barnacle—Chathamulus stellatus, Balanus glandula (Connell 1961, 1970); the bivalves—Gemma gemma (Weinberg 1985) and Tapes phillipinarum (Yap 1977); the gastropods—Conus pennaceus (Perron 1983) and Nucella lapillus (Frank 1969), and some others. These life tables contain information on both age-specific fecundity and mortality rates in local populations (or beds) as relatively dense aggregations of the species formed in typical habit.
Two methods are used to construct life tables. The first uses detailed observations of individual generations during the entire life cycle. The generation is considered as a cohort (group of individuals all born in the same time period). Life tables constructed this way are called cohort life tables. The construction of these tables is only possible when there is a real opportunity to follow a relatively large sample of individuals from the generation. The cohort life tables are extremely hard to build for populations of long-living mobile organisms. That is why the majority of these life tables were constructed for sessile species such as barnacles Balanus glandula (Connell 1970). And even in this particular case, the regular observations should have been performed for 10 years. The second is called a static life table, which is made from data collected from all ages at a particular time. It assumes the age distribution is stable from generation to generation. Static life tables are widely used in various demographic researches (Yusuf et al. 2014). However, construction of these tables is based on some assumptions: annual age-specific death rates do not change over time and annual number of births remains constant over time; thus, the population is stationary (Begon et al. 1996; Yusuf et al. 2014). The only problem is that a stationary age structure is rarely observed in marine organisms. In particular, we have not found any stationary local populations of bivalve mollusc during our long-term observations at the White Sea (Gerasimova and Maximovich 2013).
The bivalve Mya arenaria L. is a common species within soft sediment in the boreal Atlantic. In the White Sea, these clams can form dense clusters on the silty-sand intertidal beaches (Sadykhova 1979; Maximovich and Guerassimova 2003). Adult specimens do not change their habitat and are able to dig into sediments to 40–50 cm, which significantly reduces their vulnerability to predators (Sveshnikov 1963). This makes the M. arenaria beds a convenient model to study the structure and dynamics of bivalve populations.
Studies on the mortality and survival patterns of Mya arenaria have been carried out repeatedly in populations mostly limited to the North America Atlantic coast (Brousseau 1978; Commito 1982; Brousseau and Baglivo 1988; Beal et al. 2001; Beal and Kraus 2002; Beal 2006a, b). This happened largely due to the fact that Mya arenaria is considered as commercial species in this area (Brousseau and Baglivo 1988; Beal and Kraus 2002). In most cases, the studies were based on short-term field observations, of no more than 3–4 years (Brousseau 1978; Commito 1982; Brousseau and Baglivo 1988) and on even shorter experimental field studies (several months) (Beal et al. 2001; Beal 2006a, b). It was noted that the highest mortality was observed in the first years of life, while the next ontogenetic stages were characterized by a relatively high survival (Brousseau 1978; Commito 1982; Brousseau and Baglivo 1988). Most researchers pointed that predation was the main reason for high mortality at early stages (Beal et al. 2001; Strasser and Günter 2001; Beal and Kraus 2002; Strasser 2002; Flach 2003; Strasser et al. 2003; Beukema and Dekker 2005; Beal 2006a; Bowen and Hunt 2009). Along the Atlantic coast of North America, the most common predators of juvenile Mya are gastropod Euspira heros and crustacean Carcinus maenas (Commito 1982; Beal et al. 2001; Beal 2006a, b). In the Wadden Sea, they were shrimp Crangon crangon and crab Carcinus maenas (Beukema and Dekker 2005; Strasser 2002; Strasser et al. 2003; Strasser and Günter 2001). As a result, an opinion was formed, supported by experimental field studies, that is predators play a major role in regulating numbers of M. arenaria, whereas other factors, such as intraspecific relationships, are of secondary importance (Beal et al. 2001; Beal and Kraus 2002; Beal 2006b).
In the White Sea, long-term observations of several soft-shell clam beds were performed (Maximovich and Guerassimova 2003; Gerasimova and Maximovich 2013). The peculiarity of this species is the existence of beds formed by virtually a single generation for a number of years. The longest observations, since 1980, have been made at intertidal location at the Lebyazhya bight (Kandalaksha Bay). From 1989 to 2002, this bed consisted of representatives of virtually a single generation—molluscs that settled in 1988. We were able to follow this generation practically without any interruption from 1989 to 2013 (for 25 years).
In 2003, we analysed the mortality rate of the 1988 generation (Maximovich and Guerassimova 2003) for the period from 1989 to 1999. We assumed a U-shaped relationship between mortality and age (or size) where the death rate is relatively high for juveniles and older adults compared with the rest of the age groups. Further research has shown the life cycle of M. arenaria in 1999 was far from its completion. Here, we report on the survival dynamics of the same generation (up to 25 years), and test the assumption of a U-shaped relationship between mortality and age. Thus, the main purpose of this study is the analysis of age-specific mortality and survivorship in soft-shell clam beds.
In contrast to other studies on the population dynamics of soft-shell clams (Brousseau 1979; Commito 1982; Möller and Rosenberg 1983; Brousseau and Baglivo 1988; Emerson et al. 1988), our investigation was carried out in a more northerly climate, the White Sea, and it covered nearly the entire life cycle of M. arenaria. Besides, we do not have data which state that predators play a major role in regulating Mya bed densities in this biotope (Maximovich and Guerassimova 2003).
Materials and methods
The work was carried out at the Marine Biological Station of Saint Petersburg State University (MBS St.P.S.U., Fig. 1), located at the Chupa Inlet mouth (Kandalaksha Bay, the White Sea). Mya arenaria bed under consideration was allocated in the lower intertidal zone (+20–30 cm) of the silty-sand beach in the half-closed bight Lebyazhya (lat. 66°17′N; long. 33°35′E) (Fig. 1, Maximovich and Guerassimova 2003). In the Kandalaksha Bay, the amplitude of the tide does not normally exceed 2–2.5 m (Berger et al. 2001). Because of a nearby river mouth, the surface water salinity was relatively low, no more than 14–17 ‰ in summer time. Observations were carried out annually from 1989 to 2013 (except 2005 and 2009) between late June and late July.
Study area. The circle marks the investigated location
Sampling
Because of the different burrowing depth of M. arenaria of different age (and size), the sampling was done separately for specimens <20 and >20 mm (Maximovich and Guerassimova 2003). Specimens smaller than 20 mm were collected using frames of 0.01–0.1 m2 catching square depending on clam abundance. Frame size was chosen in such way that on average, samples included no less than 10 individuals each. At least 10 samples were taken on each sampling date. Sediments were excavated by a spoon from 5 cm depth and then washed out through a sieve of 1 mm mesh size. Frames of 0.25–1 m2 catching square were used to sample larger-size clams (>20 mm in shell length). The choice of frames was based on the same consideration as in small-size molluscs. Again a minimum of 10 samples were taken on each sampling time. Sediments were excavated by a spade from 30 cm depth. A total of 208 samples using frames of 0.01–0.1 m2 catching square and 232 samples using frames of 0.25–1 m2 catching square were collected during the investigations.
Processing of the samples
Molluscs from each sample were counted, and their shell length was measured to the nearest 0.1 mm. The numbers of molluscs of both size groups (smaller and larger than 20 mm) were calculated per square metre and then pooled together to give the total density at each sampling site. Mean number (N x , ind. m−2) of 1988 generation, standard error (\(m_{N} = {\raise0.7ex\hbox{$s$} \!\mathord{\left/ {\vphantom {s {\sqrt n }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\sqrt n }$}},\) where s–standard deviation, n–number of samples) and standard error percentage \(\left( {m^{{\prime }} = {\raise0.7ex\hbox{${m_{N} }$} \!\mathord{\left/ {\vphantom {{m_{N} } {N_{x} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${N_{x} }$}} \times 100} \right)\) were determined each sampling time.
Individual weight of each Mya specimen was calculated using the equation that expressed relationship between mollusc length (L) and weight (W): W =aL b . The equation parameters a and b were determined using 70 individuals of Mya arenaria collected in 2011, weighed to the nearest 0.001 g and measured to the nearest 0.1 mm. We could not use the previously calculated equation for the White Sea Mya: W = 0.00018*L 2.7 (Maximovich 1978), since it significantly underestimates individual mass in comparison with the actual data. Constants "a" and "b" of the equation are known for Mya from different parts of Atlantic North America coast (Newcombe and Kessler 1936) and the southern Baltic (Schaffer and Zettler 2007; Filippenko and Naumenko 2014, in press) (Table 1). However, due to significant climatic differences between the studied areas, we have decided to determine the equation parameters for the White Sea Mya anew.
The theoretical individual weights determined using the equation were summarized and were calculated per square metre. As a result, both total Mya biomass and the biomass of the 1988 cohort were determined on each sampling date.
Bivalve age was determined by counting shell annual growth marks. Age determination on the basis of external shell morphology is known to be rather troublesome (MacDonald and Thomas 1980; Appeldoorn 1983). Nevertheless, according to many authors (Newcombe and Kessler 1936; Swan 1952; Feder and Paul 1974), the structural marks on M. arenaria shells do reflect annual growth patterns. Some peculiarities of the studied Mya bed have allowed us to check the correctness of age determination on the basis of external shell morphology (Maximovich and Guerassimova 2003; Maximovich and Gerasimova 2004). Since 1989, a single year-class prevailed in the mollusc bed. Hence, throughout all the period of observation, we knew the exact age of molluscs of the dominating group (1988 generation). We could thus assess the reliability of age determination on the basis of shell morphology analysis. It was determined that the first growth mark (corresponding to the growth delay during the first winter after settlement) was not visible in specimens older than 2+. Taking this into consideration and counting the number of visible "growth rings" on the shells, it was possible to identify reliably individuals of 1988 generation in every sample and determine their number (N x ) and average size (L x ). Using these data, the von Bertalanffy equation was applied for growth reconstruction in 1988 generation:
$$L_{x} = L_{\infty } (1 - \exp^{{ - k(x - t_{0} )}} )$$
where L x is the shell length (mm) at a time x (years); L ∞, k and t o are constants.
Life table constructing
The life table for soft-shell clams was formed on the basis of changes in density of the 1988 cohort.
Estimates of the major demographic parameters of a life table were made according to Fedorov and Gilmanov (1980), Pianka (2000), Solbrig and Solbrig (1981), Odum (1986), Gilyarov (1990), Begon et al. (1996), and others. We analysed only data on 1988 generation density for a number of years. The reproduction characteristics of soft-shell clams in the location studied (fecundity, the minimum age/size of mature individuals, sex ratio) were not considered. The first column of the life table (x) represents bivalve age, and the second column (N x ) gives the number of living bivalves at the beginning of each age group. From these data, we could calculate several life history features. First, the proportion surviving to each life stage (l x ) was found by dividing the number of individuals living at the beginning of each age (N x ) by the initial number (N 0). N 0 is the initial density of the 1988 cohort sampled in 1989 (1 year old; 2156 ind. m−2). The proportion of the original cohort dying during each age (d x ) was found by subtracting l x+1 from l x . The age-specific mortality rate (q x ), the fraction of the population dying at each age, was calculated by dividing dx by l x . Average life expectancy of x-year-old individuals (e x ) was also estimated for each life stage of 1988 generation. Life expectancy is a useful way of expressing the probability of living "x" years beyond a given age. For the e x calculation, two more intermediate parameters were determined
- 1.
L x –average number of individuals which were alive between age x and age x + 1 and
- 2.
\({\text{T}}_{\text{x}} :L_{x} = \frac{{l_{x} + l_{x + 1} }}{2};\quad T_{x} = L_{x} + L_{x + 1} + \cdots + L_{x + n}\).
Then \(e_{x} = \frac{{T_{x} }}{{l_{x} }}\).
Results
Mya arenaria bed structure in the lower intertidal zone of the bight Lebyazhya in 1989–2013
Between 1989 and 2001, the M. arenaria bed at the sampling site consisted of virtually the only generation, settled in 1988 (Maximovich and Guerassimova 2003; Gerasimova and Maximovich 2013) (Fig. 2). In 1989, the density of the 1988 cohort was 2,156 ind. m−2, while the total density of all other generations was 16 ind. m−2. Until 2001, no one-year-old specimens have been detected at the location (Gerasimova and Maximovich 2013). Since 2001, the M. arenaria bed has been gradually transforming from a virtually single-cohort into a multi-cohort bed (Gerasimova and Maximovich 2013). Due to the lack of a significant recruitment event for 13 years, it was relatively easy to distinguish the individuals of 1988 generation from other molluscs throughout the whole study period.
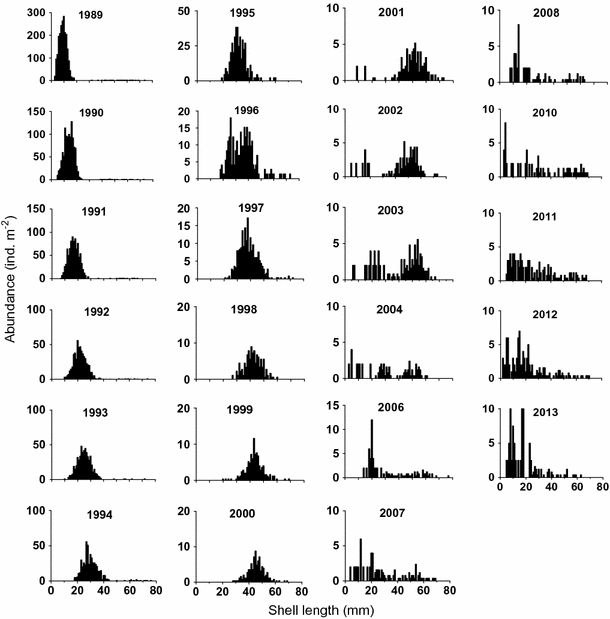
Length–frequency distribution of Mya arenaria at the low intertidal zone in the Lebyazhya bight in 1989–2013. The data of 1989–2010 from Gerasimova and Maximovich (2013)
Standard error percentage of this generation number did not exceed 30 % over a long period (until 2006) and was 40–50 % in 2006–2013. Thus, we have reasonably reliable estimates of the 1988 cohort density on each sampling date.
Dynamics of number and biomass, length of growth of Mya arenaria of 1988 generation
Abundance of separate M. arenaria year-classes varied over a wide range (Fig. 3, Table 2). The sharpest annual abundance decline (50–70 %) occurred in 1990 (for 2-year-old molluscs) and in 2004 (16-year-old molluscs). At other times during the 25-year sampling, 5–40 % of the 1988 cohort was eliminated so that by 2013, the density of that cohort was only 2 ind. m−2. Some stabilization of the 1988 cohort abundance was observed in 1992–1995 (4- to 7-year old molluscs), in 1998–2003 (10- to 15-year old molluscs) in 2004–2011 (16- to 23-year old molluscs).
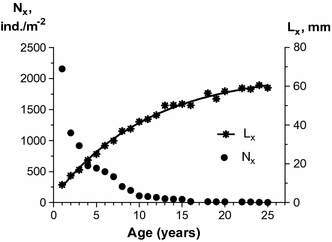
The number (N x ) variation by age and growth length reconstruction (L x ) of the 1988 cohort of Mya arenaria
Individual weight of each Mya specimen of the 1988 cohort was calculated, and the original equation expressed relationship between clam length and weight (Fig. 4, Table 1).
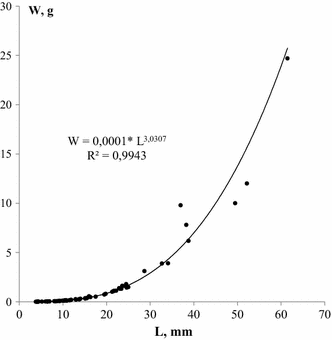
Length (L) versus weight (W) of Mya arenaria, sampled in the low intertidal zone of Lebyazhya bight, 2011
Analysis of the total biomass dynamics of the 1988 cohort during the study period showed that there was no complete resemblance in the dynamics of biomass and density in M. arenaria bed. The period of abundance stabilization in 4- to 7-year old molluscs coincided with a period of steady growth of biomass (Fig. 5). As a result, in 1995 (7-year-old molluscs), their biomass reached its maximum for the entire period of observation, almost 1.6 kg/m2. Total biomass of Mya arenaria at the location was highest in 1994–1996, about 2.0 kg/m2. From 1995 to 1998, biomass declined and has remained relatively stable at 1 kg m−2 until 2003, when the cohort was 15 years old. In 2004, biomass of the 1988 cohort decreased by almost 4 times, and the next 7 years (until 2011), it remained around 250–300 g/m2.

Biomass (B, g/m2) variation of the 1988 cohort of Mya arenaria by age
The study of M. arenaria growth was not the primary objective of the study; however, since mean clam length of the 1988 cohort was estimated on each sampling date since 1989, it was possible to evaluate growth rates of this group (Fig. 3). The period of highest growth rate was observed in the first 5 years of life (of about 5 mm per year). At the age of 6–9 years, molluscs still retained a significant growth rate (of about 3 mm per year). But for animals between 10 and 15 years, the average value was less than 2 mm per year. The lowest growth rate (less than 1 mm per year) was observed in molluscs older than 15 years. The maximum observed size of Mya arenaria in 1988 generation was 71 mm (at age of 20 years).
Life table of Mya arenaria: age-specific survival and mortality rate
The life table for Mya arenaria (Table 2) was constructed on the basis of changes in density of the 1988 cohort that were observed on each sampling date. Data on survival of the 1988 cohort (l x , Table 2) were used to develop a survival curve that was approximated by an exponential model (Fig. 6). Mortality rate (q x ) of 1988 generation varied more than tenfold (from 0.06 to 0.68 year−1) over the study period (Table 2). No other regular changes in this index were observed (Fig. 7). Periods of relatively high survival alternated with a sharp increase in mortality:
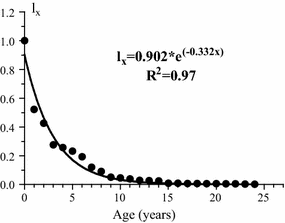
Survivorship curve of the 1988 cohort of Mya arenaria. l x proportion surviving to each life stage
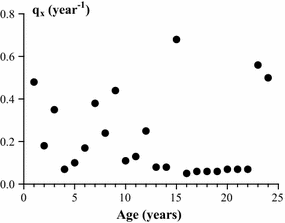
The mortality rate (q x , year−1) of the 1988 cohort of Mya arenaria by age
- 1.
Mortality rate was the highest at age of 1 year (0.48 year−1), 7–9 years (0.38–0.48 year−1), 15 years (0.68 year−1) and older than 22 years (0.5–0.56 year−1).
- 2.
Mortality rate was relatively low at age of 4–6 years (0.07–0.17 year−1), 10–14 years (0.08–0.25 year−1) and 16–22 years (0.05–0.07 year−1).
Dynamics of life expectancy (e x ) was in large degree similar to the dynamics of mortality rate. The minimum values of life expectancy, about 2–3 years, were observed during periods of high mortality rates, and the maximum values in periods of low mortality. For example, for 16- to 19-year old molluscs (in 2004–2007) e x reached 4.4–6.4 years.
Discussion
Mya arenaria are found almost everywhere in soft sediments in the coastal zone of the White Sea and are capable of forming massive clusters on intertidal flats (Russanova 1963; Sveshnikov 1963; Maximovich and Guerassimova 2003). One such typical habitat for the soft-shell clams is silty-sand beach in the intertidal zone of Lebyazhya bight, where the material was collected for this study. The present work was in great extent prompted by specific dynamics of the studied Mya arenaria bed—for many years (over 11 years), the bed consisted of representatives of virtually a single generation (in this case the 1988 cohort) with practically no traces of annual recruitment (Gerasimova and Maximovich 2013). However, long-term changes in Mya bed structure in the Lebyazhya bight were quite similar to that in other areas. Sharp dominance of individual generations for several years proved to be true for other beds both in the White Sea (Sadykhova 1982; Gerasimova and Maximovich 2013) and in other parts of their geographic range (Commito 1982; Strasser et al. 1999, 2003). The predominance of a single generation was explained mainly by interannual shifts in recruitment level (Commito 1982; Strasser et al. 1999, 2003; Flach 2003; Bowen and Hunt 2009). Causes of fluctuations in recruitment level of Mya arenaria have been repeatedly discussed by different researchers including the authors of this study (Brousseau 1978; Commito 1982; Sadykhova 1982; Günter 1992; Maximovich and Guerassimova 2003; Gerasimova and Maximovich 2013). For the White Sea soft-shell clam beds (in particular, for those in the Lebyazhya bight), interannual fluctuations in juvenile numbers were mainly explained (Maximovich and Guerassimova 2003; Gerasimova and Maximovich 2013) by the competitive relationship between adults and juveniles known for bivalves (Möller and Rosenberg 1983; Olafsson 1989; Günter 1991, 1992) and by survival peculiarities of spat in their first winter (Kühl 1951; Strasser et al. 2001; Bowen and Hunt 2009). This conclusion was based on the following facts: (1) favourable environmental conditions for reproduction of this species in the White Sea and the annual presence of larvae in summer plankton (Maximovich and Shilin 2012); (2) coincidence in time of significant juvenile number in beds and substantial (Maximovich and Guerassimova 2003; Gerasimova and Maximovich 2013); (3) asynchronous development of different Mya arenaria beds in the White Sea, i.e. in the same time different beds were dominated by different generations (Gerasimova and Maximovich 2013); (4) significant spat mortality (up to 100 %) during the first year of life (Gerasimova and Maximovich 2013). Besides, the lack of juveniles in Mya arenaria beds in the White Sea could be related to the impact of predators. At the studied area, birds (the gulls (Larus argentatus, Larus canus), the catcher, Haematopus ostralegus, the ruddy turnstone, Arenaria interpres, the common crane, Grus grus, the common eider, Somateria mollissima), fishes (the Atlantic cod, Gadus morhua,, the flounder, Pleuronectus flesus, the butterfish, Pholis gunnellus, guffer, Zoarces viviparous), and starfish, Asterias rubens, (Shklyarevich and Shcherbakova 2005) could be the potential predators for young molluscs not borrowing deeper than 5–8 cm.
It should be noted that the observed long-term changes in density of the 1988 cohort in the Mya arenaria bed in the Lebyazhya bight apparently have been due to natural causes. At the studied area, a commercial or recreational fishing for this species does not exist. Practically, we only collected the soft-shell clams during the whole time of the investigation (1989–2013). Besides, we observed neither extreme events in the intertidal zone nor significant deviations in climatic or hydrological characteristics in the studied area in 1989–2013.
Mya arenaria survivorship and mortality
We observed relatively high variation in mortality rates of M. arenaria through time at the study site, which is similar in other bivalve populations (Brousseau 1978; Freeman and Dickie 1979; Ankar 1980; Commito 1982; Josefson 1982; Brousseau and Baglivo 1988; McGrorty et al. 1990). For many populations of aquatic invertebrates and marine fish, the most adequate model was survivorship curve Type III, where mortality is highest at the youngest ages, and decreases as the population ages (Deevey 1947; Begon et al. 1996). The survival of the 1988 cohort fits this model.
High mortality rates of Mya arenaria at early stages have been repeatedly observed (Beal et al. 2001; Strasser and Günter 2001; Beal and Kraus 2002; Strasser 2002; Flach 2003; Strasser et al. 2003; Beal 2006a; Beukema and Dekker 2005; Bowen and Hunt 2009). Most researchers attribute this fact to epibenthic predators such as the moon snail, Euspira heros, the shore crab, Carcinus maenas, or the sand shrimp, Crangon crangon. Thus, due to predation of Euspira heros, Mya survival at the North America Atlantic coast during the first 5 years of life does not exceed 3.5 % (Commito 1982). In the Wadden Sea, a successful recruitment of Mya arenaria and other bivalve species (Cerastoderma edule, Macoma balthica) was observed after severe winters, which was associated with a decreased activity of epibenthic predators (Strasser and Günter 2001; Strasser 2002; Strasser et al. 2003). Thus, many researchers (Beal et al. 2001; Strasser and Günter 2001; Beal and Kraus 2002; Strasser et al. 2003; Beal 2006b) have considered predation as the main biotic factor, while other factors, including intraspecific relationships, as secondary factors (Beal et al. 2001; Beal 2006b). The experimental study in Maine, USA, along the northwestern Atlantic coast, showed statistically significant increases in mortality in dense clusters of soft-shell clam juveniles (Beal et al. 2001; Beal 2006b). However, the researchers believed it is unlikely that reduced survival in dense Mya beds was due to starvation, as it was found very few numbers of dead clams with undamaged valves (Beal et al. 2001). Previously, we pointed out that at our study site (the Lebyazhya bight), shrimps, Crangon crangon, as well as other predators (crabs, gastropods, starfish, flounder) were not represented or were scarce (Maximovich 1989; Maximovich and Guerassimova 2003). However, the observations occurred during a short period of time each year (June–July) and that no attempt has been made using an experimental approach with juveniles of soft-shell clam to estimate directly mortality rates due to biotic or abiotic factors. Therefore, we cannot exclude the impact of predators (birds, fishes) on M. arenaria young molluscs. Nevertheless, significant number of dead clams with undamaged valves (including those of less than 20 mm length) observed throughout the study period (personal observation of Gerasimova A.V.) suggests that this was not the case. It is possible that at low predation pressure (or lack of it), the major role in regulating Mya arenaria bed density belongs to other biotic factors, such as intraspecific relations. Also, in the early stages of development (especially in the first winter after settling) when molluscs inhabit surface sediment layer, severe winter conditions, for example, abrading action of ice, may also impact juvenile survival (Kühl 1951; Strasser et al. 2001; Bowen and Hunt 2009). According to observations by Kühl (1951), in 1947/1948, the winter ice cover in the Wadden Sea has resulted in 100 % soft-shell clam spat elimination. The same phenomena (100 % spat elimination) we observed after the first winter after settling in the White Sea Mya beds (Gerasimova and Maximovich 2013).
Many researchers often associate increased survival and reduced vulnerability of Mya arenaria to predators with increasing age and size (Brousseau 1978; Commito 1982; Brousseau and Baglivo 1988). For example, it was shown that Mya is the subject of attack by predatory snails Euspira heros only to a size of 30 mm (Commito 1982). It should be noted that according to our data, relatively high rate of mortality was observed not only in the first years of life. We were able to show that separate stages of M. arenaria life cycle differed substantially in vulnerability. Apparently, the assumed U-shaped relationship between mortality and age (Maximovich and Guerassimova 2003) was not suitable for describing the dynamics of Mya single cohort throughout the life cycle. Although such U-shaped relations are known from other investigations (Alimov 1989), it was not the case for the Mya bed sampled in this study. During our observation, the periods of low mortality alternated with a much higher rate of mortality. Comparison of variability in mortality rate with the cohort biomass dynamics allows the following interpretation.
High mortality rate of molluscs during the first years of life might be due to intense intraspecific relationships in dense aggregations of Mya young molluscs. Stiff competition for food and space, obviously, leads to a rapid drop in the juvenile abundance down to optimum values. Then, a stabilization of mollusc number takes place. We have too little data for statistical analysis of density impact on soft-shell clam survival. Nevertheless, in studied Mya bed in the lower intertidal zone, the number of two-year-olds decreased almost two times compared to their amount in the previous season (2,156 ind. m−2 in 1989 and 1,124 ind. m−2 in 1990). In the middle intertidal zone, the number of yearlings in 1989 was almost 2.5 times less than that in the lower zone, and the statistically significant differences between mollusc density in 1989 and in 1990 were not observed (818 ind. m−2 in 1989 and 814 ind. m−2 in 1990) (unpublished data). Without experimental studies, we could not exclude potential predators' impact on the survival of Mya young molluscs (under 4 years of age and of less than 20 mm length). But this possible predator impact could not explain the differences in mortality rate of yearlings in the middle and low intertidal zones, differed in Mya abundance. And this possible predator impact could not explain relatively low mortality rate (0.18 year−1) of two-year-old clams in the low intertidal zone where they should most probably fall under predator impact. In addition, as it was already noted, the high mortality rate of young individuals was possibly due to their habitation in the surface sediment layer, i.e. in a rather dynamic environment. In these conditions, young molluscs could die due to their non-viability. It is partly supported by the data that in Mytilus edulis larvae pool in the Chupa Inlet (The White Sea), a number of individuals have visually distinct developmental disorders (Kulakovsky and Flachinskaja 1993). Severe winter conditions could also have negative impact on the number of young clams (e.g. abrading action of ice) (Kühl 1951; Strasser et al. 2001; Bowen and Hunt 2009).
Relatively low mortality rate of 4- to 6-year old Mya could be the result of the mollusc greater protection (due to burrowing as deep as 5–10 cm) and improved balance between the amount of available food and nutritional needs. However, this period was also a period of molluscs active growth (about 4 mm per year), resulting in a sharp increase in generation biomass. The biomass of 7-year-old individuals apparently reached its capacity for this environment (1.5 kg m−2). A further increase in mortality rate of the 1988 cohort may reflect, for example, increased competition for food among grown animals.
The next stage of the cohort showed a stabilization of density from ages 10–15 years. This was apparently the result of setting the balance between environmental capacity and mollusc food demands. M. arenaria mean growth rate was less than 2 mm per year in this period. Moreover, clams could dig to a depth of 30–40 cm, and thus become inaccessible to predators. The sharp increase in mortality rate at the age of 15 years was probably due to the average life expectancy the majority of the representatives of 1988 cohort achieved. Less than 1 % of the 1988 cohort, in relation to their density reported in 1989, overcome 15-year age limit. After that the group of few long-living animals was formed who had low growth rate (about 1 mm per year). They had reached the age of 23–25 years, probably the maximum age for M. arenaria in the White Sea, in almost unchanged composition.
Lifespan and length growth of Mya arenaria
Although not the main objective of this work, we were able to estimate the lifespan and growth rate (length) of M. arenaria from this White Sea population. The average lifespan and likely maximum possible lifespan was 15 and 24–25 years, respectively. The data on the maximum lifespan of Mya arenaria exceeded significantly the previously reported estimates for the White Sea soft-shell clams (from 7–8 to 17 years), obtained by different researchers, including the authors of this article (Maximovich 1978, 1989; Sadykhova 1979; Maximovich and Guerassimova 2003; Shklyarevich and Shcherbakova 2005). We believe that previously the lifespan was underestimated mainly due to the difficulties in Mya arenaria age determination using external shell morphology (Maximovich and Gerasimova 2004). As a result of the analysis of the growth rings on the external surface of the valves, we observed two items that should be noted for future studies of this species in the White Sea: (1) the lack of clear, first-year growth lines due to erosion of the shell near the hinge; and (2) the convergence of growth lines formed during the later stages of the life cycle. In the studied Mya bed, the maximum size of an individual from the 1988 cohort was 71 mm (at the age of 20 years) and the maximum lifespan was at least 25 years. These estimates fit well into the known range of similar characteristics of the same species from different geographic locations, where lifespan varied from 4 to 28 years, while maximum length ranged from 27 to 150 mm (Feder and Paul 1974; Warwick and Price 1975; Evans and Tallmark 1977; MacDonald and Thomas 1980; Commito 1982; Emerson et al. 1988; Appeldoorn 1995; Cardoso 2007). However, the Mya arenaria growth rate in the studied bed was significantly lower than that in other areas of the species distribution, especially at the Atlantic coast of North America. Thus, in our studies, Mya arenaria reached an average size of 42 mm in 10 years, while in the mid-Atlantic Mya arenaria reached an average size of 150 mm in 8 years (Abraham and Dillon 1986), in the southern Baltic Sea 40 mm in 5 years (Filippenko and Naumenko 2014). The reason may be the severe temperature conditions and low salinity in the study area (Maximovich and Guerassimova 2003). Negative water temperatures (down to −1.5 °C) were observed in the study area from late November to late April, while in the summer, the 2 m upper water layer warms up to 15–17 degrees. Because of the study area proximity to the river Keret mouth, the summer salinity was no more than 14–17 ‰. It is interesting that almost the same rate of length growth was reported by Commito (1982) for the Mya arenaria of the Cobscook Bay (Maine, USA) where the water temperature and salinity were very close to that in the area we have studied: seasonal changes in surface water temperature was 0–10 °C, salinity was usually about 30 ‰, but could drop as low as 15 ‰. Here, the growth rate of soft-shell clams in the first 5 years was about 5 mm per year, and the average 4-year-old clams reached 20 mm. It is known that growth rate of Mya arenaria depends on temperature and salinity regime (Brousseau 1979; Appeldoorn 1983), sediment type (Swan 1952; Newell and Hidu 1982; Martynov et al. 2007) and nutritional conditions (Newell and Hidu 1986; Roseberry et al. 1991; Beal et al. 2001; Carmichael et al. 2004; Beal 2006b). Also, the effect of intraspecific density on Mya arenaria growth rate is described in the literature (Newell and Hidu 1986; Strasser et al. 1999; Beal et al. 2001). But detailed analysis of M. arenaria growth in the White Sea will be the subject of our future work.
Conclusion
We describe the mortality and growth rate of a cohort of soft-shell clams from a low intertidal location in the White Sea over a 25-year period. The Mya arenaria bed was located in a typical habitat for this species, and the patterns of the population dynamics were consistent with that in other sites in this region. Therefore, there is a reason to assume that the revealed patterns of Mya survival and mortality are typical not only for the studied bed, but are more widely spread.
On the basis of our data, we could suggest the possible reasons for the increase of Mya arenaria mortality rate during its life cycle:
- A.
living in the surface sediment layer at early stages of life cycle (unstable environment, high mortality of non-viable clams, predator impact)
- B.
intense intraspecific relationships in dense aggregations of young clams;
- C.
increased intraspecific competition during periods of the rapid individual growth;
- D.
ageing (clam mortality increases with older age–achievement of an average and a maximum lifespan).
References
-
Abraham BJ, Dillon PL (1986) Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic): soft-shell clam. Biological report, Washington, DC, vol 82 (11.68)
-
Alimov AF (1989) Introduction to production hydrobiology. Hydrometeoizdat, Leningrad. (in Russian)
-
Ankar S (1980) Growth and production of Macoma balthica (L.) in a northern Baltic soft bottom. Ophelia Supplementum 1:31–48
-
Appeldoorn RS (1983) Variation in the growth rate of Mya arenaria and its relationship to the environment as analyzed through principal component analysis and the ω parameter of von Bertalanffy equation. Fish Bull 81:75–85
-
Appeldoorn RS (1995) Covariation in life history parameters of soft-shell clams, Mya arenaria along a latitudinal gradient. In: ICES marine science symposia, pp 19–25
-
Beal BF (2006a) Biotic and abiotic factors influencing growth and survival of wild and cultured individuals of the soft-shell clam (Mya arenaria L.) in Eastern Maine. J Shellfish Res 25:461–474
-
Beal BF (2006b) Relative importance of predation and intraspecific competition in regulating growth and survival of juveniles of the soft-shell clam, Mya arenaria L., at several spatial scales. J Exp Mar Biol Ecol 336:1–17
-
Beal BF, Kraus GM (2002) Interactive effects of initial size, stocking density, and type of predator deterrent netting on survival and growth of cultured juveniles of the soft-shell clam, Mya arenaria L., in eastern Maine. Aquaculture 208:81–111
-
Beal BF, Parker MR, Vencile KW (2001) Seasonal effects of intraspecific density and predator exclusion along a shore-level gradient on survival and growth of juveniles of the soft-shell clam, Mya arenaria L., in Maine, USA. J Exp Mar Biol Ecol 264:133–169
-
Begon M, Harper JL, Townsend CR (1996) Ecology: individuals, populations, and communities, 3rd edn. Blackwell Science
-
Berger V, Dahle S., Galaktionov K, Kosobokova X, Naumov A, Rat'kova T, Savinov V, Savinova T (2001) White sea. Ecology and environment. St-Petersburg, Tromso
-
Beukema JJ, Dekker R (2005) Decline of recruitment success in cockles and other bivalves in the Wadden Sea: possible role of climate change, predation on postlarvae and fisheries. Mar Ecol Prog Ser 287:149–167
-
Bowen J, Hunt H (2009) Settlement and recruitment patterns of the soft-shell clam, Mya arenaria, on the northern shore of the Bay of Fundy, Canada. Estuaries Coast 32:758–772
-
Brousseau DJ (1978) Population dynamics of the soft-shell clam Mya arenaria. Mar Biol 50:63–71
-
Brousseau DJ (1979) Analysis of growth rate in Mya arenaria using the Von Bertalanffy equation. Mar Biol 51:221–227
-
Brousseau DJ, Baglivo JA (1988) Life tables for two field populations of soft-shell clam, Mya arenaria, (Mollusca: Pelecypoda) from long island sound. Fish Bull 86:567–579
-
Cardoso JFMF (2007) Growth and reproduction in Bivalves. An energy budget approach. Dissertation, University of Groningen
-
Carmichael RH, Shriver AC, Valiela I (2004) Changes in shell and soft tissue growth, tissue composition, and survival of quahogs, Mercenaria mercenaria, and soft-shell clams, Mya arenaria, in response to eutrophic-driven changes in food supply and habitat. J Exp Mar Biol Ecol 313:75–104
-
Commito JA (1982) Effects of Lunatia heros predation on the population dynamics of Mya arenaria and Macoma balthica in Maine, USA. Mar Biol 69:187–193
-
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
-
Connell JH (1970) A predator-prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecol Monogr 40:49–78
-
Deevey ES Jr (1947) Life tables for natural populations of animals. Q Rev Biol 22:283–314
-
Emerson CW, Minchinton TE, Grant J (1988) Population structure, biomass, and respiration of Mya arenaria L. on temperate sandflat. J Exp Mar Biol Ecol 115:99–111
-
Evans S, Tallmark Bo (1977) Growth and biomass of bivalve molluscs on a shallow, sandy bottom in Gullmar Fjord (Sweden). Zoon 5:33–38
-
Feder HM, Paul AJ (1974) Age, growth and sizeweight relationships of the soft-shelled clam Mya arenaria in Prince William Sound, Alaska. Proc Natl Shellfish Assoc 64:45–52
-
Fedorov VD, Gilmanov TG (1980) Ecology. MSU, Moscow. (in Russian)
-
Filippenko D, Naumenko E (2014) Patterns of the growth of soft-shell clam Mya arenaria L. (Bivalvia) in shallow water estuaries of the southern Baltic Sea. Ecohydrol Hydrobiol 14:157–165
-
Flach EC (2003) The separate and combined effects of epibenthic predation and presence of macro-infauna on the recruitment success of bivalves in shallow soft-bottom areas on the Swedish west coast. J Sea Res 49:59–67
-
Frank PW (1969) Growth rates and longevity of some gastropod molluscs on the coral reef at Heron Island. Oecologia 2:232–250
-
Freeman KR, Dickie LM (1979) Growth and mortality of the blue mussel Mytilus edulis in relation to environmental indexing. J Fish Res Board Can 36:1238–1249
-
Gerasimova A, Maximovich N (2013) Age-size structure of common bivalve mollusc populations in the White Sea: the causes of instability. Hydrobiologia 706:119–137
-
Gilyarov AM (1990) Population ecology: teaching aid. MSU, Moscow
-
Günter C-P (1991) Settlement of Macoma balthica on an intertidal sandflat in the Wadden Sea. Mar Ecol Prog Ser 76:73–79
-
Günter C-P (1992) Settlement and recruitment of Mya arenaria L. in the Wadden Sea. J Exp Mar Biol Ecol 159:203–215
-
Josefson AB (1982) Regulation of population size, growth, and production of a deposit-feeding bivalve: a long-term field study of three deep-water populations off the swedish west coast. J Exp Mar Biol Ecol 59:125–150
-
Kühl H (1951) Uber die siedlungsweise von Mya arenaria. Verhandlungen der Deutschen Zoologischen Gesellschaft 25:358–391
-
Kulakovsky EJ, Flachinskaja LP (1993) Peculiarities of larvae development in he White Sea mussels (Mytilus edulis L.). Formation of regulatory system elements. In: Investigations of the mussel mariculture in the White Sea, St. Petersburg, pp 61–82. (in Russian)
-
MacDonald BA, Thomas MLH (1980) Age determination of the soft-shell clam Mya arenaria using shell internal growth lines. Mar Biol 58:105–109
-
Martynov FM, Gerasimova AV, Maximovich NV (2007) Length growth of Mya arenaria L. in the intertidal zone of the Keret Archipelago (Kandalakshsky Bay, White Sea). Vestnik St. Petersburg University, Series 3 Biology 1: 28–36. (in Russian)
-
Maximovich NV (1978) Peculiarities of ecology and bioenergetic traits of population of Mya arenaria L. (Bivalvia) in the Chupa Inlet. Vestnik the Leningrad University, Series 3 Biology pp 28–36. (in Russian)
-
Maximovich NV (1989) Dynamics of production traits in littoral bed of Mya arenaria L. (The Chupa Inlet, The White Sea). Vestnik the Leningrad University, Series 3 Biology 1: 19–24. (in Russian)
-
Maximovich NV, Gerasimova AV (2004) Age determination of the White Sea bivalves by the shell morphology. In: Proceedings of the V scientific session of the marine biological station of St. Petersburg State University, St. Petersburg, Russia, St. Petersburg, pp 29–30. (in Russian)
-
Maximovich NV, Guerassimova AV (2003) Life History characteristics of the clam Mya arenaria in the White Sea. Helgol Mar Res 57:91–99
-
Maximovich NV, Shilin MB (2012) Spatial-temporal distribution of Bivalve planktonic larvae in the semi-isolated waters (for example, Chupa Inlet of the White Sea). Biosphera 4:293–306 (in Russian)
-
McGrorty S, Clarke RT, Reading CJ, Goss-Custard JD (1990) Population dynamics of the mussel Mytilus edulis: density changes and regulation of the population in the Exe estuary, Devon. Mar Ecol Prog Ser 67:157–169
-
Möller P, Rosenberg K (1983) Recruitment, abundance and production of Mya arenaria and Cardium edule in marine waters, Western Sweden. Ophelia 22:33–35
-
Newcombe CL, Kessler H (1936) Variations in growth indices of Mya arenaria L. on the Atlantic coast of North America. Ecology 17:429–443
-
Newell CR, Hidu H (1982) The effects of sediment type on growth rate and shell allometry in the soft shelled clam Mya arenaria L. J Exp Mar Biol Ecol 65:285–295
-
Newell CR, Hidu H (1986) Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (North Atlantic): soft-shell clam. Biological report, Washington, DC, vol 82(11.53)
-
Odum, Y. (1986). Ecology. Vol. 1. Mir, Moscow. (in Russian)
-
Olafsson EB (1989) Contrasting influences of suspension-feeding and deposit-feeding populations of Macoma balthica on infaunal recruitment. Mar Ecol Prog Ser 55:171–179
-
Perron FE (1983) Growth, fecundity, and mortality of Conus pennaceus in Hawaii. Ecology 64:53–62
-
Pianka ER (2000) Evolutionary ecology, 6th edn. Benjamin Cummings
-
Roseberry L, Vincent B, Lemaire C (1991) Croissance et reproduction de Mya arenaria dans la zone intertidale de l'estuaire du Saint-Laurent. Can J Zool 69:724–732
-
Russanova MN (1963) Short notes on biology of some mass invertebrate species in the vicinity of the cape Kartesh. In: Materials of integrated research of the White Sea, 2, USSR Academy of Science, Moscow-Leningrad, pp 53–65. (in Russian)
-
Sadykhova IA (1979) Biological features of Mya arenaria (Mollusca, Lamellibranchia) in the White Sea. Zool J 58:804–809 (in Russian)
-
Sadykhova IA (1982) Changes in abundance and size-frequency distribution of Mya arenaria L. population in the White Sea. In: Proceedings of 1st coordination meeting "Productivity increase, sustainable use and conservation of the white sea natural resources", Leningrad, Russia, Leningrad, pp 73–74. (in Russian)
-
Schaffer F, Zettler ML (2007) The clam siphon as indicator for growth indices in the soft-shell clam Mya arenaria. Helgol Mar Res 61:9–16
-
Shklyarevich GA, Shcherbakova IB (2005) Long-term changes of Mya arenaria beds in the intertidal zone of Kandalaksha Bay (White Sea). In: Proceedings of the IXth international conference "the study, sustainable use and conservation of natural resources of the White Sea", Petrozavodsk, Karelia, Petrozavodsk, pp 327–332. (in Russian)
-
Solbrig OT, Solbrig DJ (1981) Introduction to population biology and evolution. Addison-Wesley Publishing Company
-
Strasser M (2002) Reduced epibenthic predation on intertidal bivalves after a severe winter in the European Wadden Sea. Mar Ecol Prog Ser 241:113–123
-
Strasser M, Günter C-P (2001) Larval supply of predator and prey: temporal mismatch between crabs and bivalves after a severe winter in the Wadden Sea. J Sea Res 46:57–67
-
Strasser M, Walensky M, Reise K (1999) Juvenile-adult distribution of the bivalve Mya arenaria on intertidal flats in the Wadden Sea: why are there so few year classes? Helgol Mar Res 53:45–55
-
Strasser M, Reinwald T, Reise K (2001) Differential effects of the severe winter of 1995/96 on the intertidal bivalves Mytilus edulis, Cerastoderma edule and Mya arenaria in the Northern Wadden Sea. Helgol Mar Res 55:190–197
-
Strasser M, Dekker R, Essink K, Günter C-P, Jaklin S, Kroncke I, Madsen PB, Michaelis H, Vedel G (2003) How predictable is high bivalve recruitment in the Wadden Sea after a severe winter? J Sea Res 49:47–57
-
Sveshnikov VA (1963) Biocenotic relations and environmental conditions of some food invertebrates of intertidal infauna in the Kandalaksha Bay of the White Sea. Proc Kandalaksha Reserv 4:114–134 (in Russian)
-
Swan EF (1952) Growth indices of the clam Mya arenaria. Ecology 33:365–374
-
Warwick RM, Price R (1975) Macrofauna production in an estuarine mud-flat. J Mar Biol Assoc UK 55:1–18
-
Weinberg JR (1985) Factors regulating population dynamics of the marine bivalve Gemma gemma: intraspecific competition and salinity. Mar Biol 86:173–182
-
Yap WG (1977) Population biology of the japanese little-neck clam, Tapes philippinarum, in Kaneohe Bay, Oahu, Hawaiian Islands. Pac Sci 31:223–244
-
Yusuf F, JoM Martins, Swanson D (2014) Life Tables. Methods of demographic analysis. Springer, Netherlands, pp 143–172
Acknowledgments
We are grateful to all the students and staff of the Ichthyology and Hydrobiology Department of St. Petersburg State University who have helped us a lot in mollusc sampling and data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-D. Franke.
Rights and permissions
About this article
Cite this article
Gerasimova, A.V., Maximovich, N.V. & Filippova, N.A. Cohort life tables for a population of the soft-shell clam, Mya arenaria L., in the White Sea. Helgol Mar Res 69, 147–158 (2015). https://doi.org/10.1007/s10152-014-0423-2
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1007/s10152-014-0423-2
Keywords
- Bivalvia
- Mya arenaria
- Life table
- Mortality
- Survival
- White Sea
hodgedooketherver.blogspot.com
Source: https://hmr.biomedcentral.com/articles/10.1007/s10152-014-0423-2
0 Response to "Sea Stars Feed on Marine Mollusks Survivorship Graph"
Post a Comment